

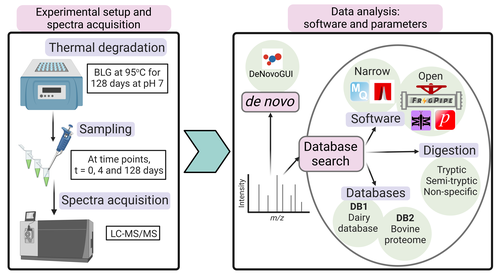
Paleoproteomics is a rapidly growing field with numerous challenges, many of which are due to the highly fragmented, modified, and degraded nature of ancient proteins. Though there are established standards for analysis, it is unclear how different software tools affect the identification and quantification of peptides, proteins, and post-translational modifications. To address this knowledge gap, Rodriguez Palomo et al. design a controlled system by experimentally degrading and purifying bovine beta-lactoglobulin, and then systematically compare the performance of many commonly used tools in its analysis.
They present comprehensive investigations of false discovery rates, open and narrow searches, de novo sequencing coverage bias and accuracy, and peptide chemical properties and bias. In each investigation, they explore wide ranges of appropriate tools and parameters, providing guidelines and recommendations for best practices. Based on their findings, Rodriguez Palomo et al. develop a proposed pipeline that is tailored for the analysis of ancient proteins. This pipeline is an important contribution to paleoproteomics and is likely to be of great value to the research community, as it is designed to enhance power, accuracy, and consistency in studies of ancient proteins.
References
Ismael Rodriguez-Palomo, Bharath Nair, Yun Chiang, Joannes Dekker, Benjamin Dartigues, Meaghan Mackie, Miranda Evans, Ruairidh Macleod, Jesper V. Olsen, Matthew J. Collins (2023) Benchmarking the identification of a single degraded protein to explore optimal search strategies for ancient proteins. bioRxiv, ver.3 peer-reviewed and recommended by PCI Math Comp Biol https://doi.org/10.1101/2023.12.15.571577
I thank Palomo et al. for their thoughtful responses to my reviews. The physicochemical section is excellent. I'm happy that you included the isoelectric point and other properties. I think it really strengthens the observed results and will be helpful for similar future preservational studies on other proteins.
 , 17 Oct 2024
, 17 Oct 2024The authors have adjusted all of the comments I requested. The paper reads well and is extremely thorough. I would recommend the manuscript at this point.
The only “issue” I have is that the proposed pipeline includes several searches using multiple platforms for each sample, which is exponentially increases the time and work to complete any study. Hopefully some researchers incorporate this pipeline into new work so we can see the how much these additional steps add to the reliability of ancient protein studies.
DOI or URL of the preprint: https://doi.org/10.1101/2023.12.15.571577
Version of the preprint: 2
The submitted manuscript is an important contribution to paleoproteomics. However, the reviewers have identified several issues with the clarity of the manuscript text and figures. I recommend that the authors carefully address these on a point-by-point basis, particularly as this manuscript should serve as a guide for best practices in paleoproteomics.
Download the review , 22 Jul 2024
, 22 Jul 2024Please see my attached document with comments. Please feel free to contact me with any questions. Apologies if anything isn't clear - I just returned from holiday am down with COIVD...
Shevan
Download the review